Calculate the Kc Value for the a -protein Binding Reaction.
Prev Question Next Question 0 votes. How do you calculate kc for a reaction.

13 4 Equilibrium Calculations Chemistry
Express your answer using two significant figures.

. Get the detailed answer. How do you calculate kc for a reaction. Complete the equations for the following equilibria calculate Kc for each reaction and express its value in proper scientific notation.
G 0 and Qc Kc or Kp. Calculate the value of Kc for the reaction 2 N 2 Og 3 O 2 g 2 N 2 O 4 g using. Thank you in advance.
Calculate the value of kc for the reaction 2 n 2 og 3. This question success to consider this generic reaction where a goes to B and C and asked us to find the equilibrium concentrations of A B and C for three diff. T is temperature in Kelvin.
The spontaneity of the process is related to the free energy change. Please help I am so lost. Click Save and Submit to save and submit.
As an example we will calculate Kc for two. Course Title BIO 97. The formula is below.
University of California Irvine. Students who viewed this also studied. Need help with chemistry.
How do you calculate the KC of a reaction. How do you calculate kc for a reaction. Brackets denote reagent concentrations that must be given in order to compute Kc.
The following reaction occurs and equilibrium is established. Assuming that the drug that binds more strongly will be more effective which drug is the better choice for further research. The reaction will proceed to form products.
On this page we have gathered for you the most accurate and comprehensive information that will fully answer the question. A2BC At equilibrium A 0430M and C 0470M. At equilibrium the concentration of D was 0500 M.
2 August 2021 - A2 Chemistry - Equilibrium Constant Kc - How to Find Kc - Kc Value - Calculate KcEquilibrium Constant KcThe equilibrium constant of a chemica. The Q value can be compared to K to determine the direction of the reaction to take place. Calculate the value of the equilibrium constant Kc.
For the reaction ABAB the equilibrium constant Kc is defined as ABAB. G 0 and Qc Kc or Kp at the start of the reaction. Calculate a value for the concentration of.
QUESTION 8 Calculate the value of Kc for the reaction in question 7 at 250C QUESTION 9 Consider the reaction 2 AB 3C D. Are you trying to find the equation. Calculate the value of Kc.
The letter c implies that reagent amounts are expressed as molar concentration. Complete the equations for the following equilibria calculate Kc for each reaction and express its value in proper scientific notation. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators.
Pages 8 This preview shows page 6 - 8 out of 8 pages. Asked Jun 2 2018 in Chemistry by Golu 106k points Calculate the value of K c for the reaction. How to calculate kc and kp.
If the question gives you starting concentrations and equilibrium concentrations of. After equilibrium was reached the concentration of I2 was. Calculate value for Kc given equilibrium concentrations given Kc calculate from CHEMISTRY 1301 at University of Texas Dallas.
This question success to consider this generic reaction where a goes to B and C and asked us to find the equilibrium concentrations of A B and C for three diff. This page explains equilibrium constants expressed in terms of partial pressures of gases K pIt covers an explanation of the terms mole fraction and partial pressure and looks at K p for both. Calculate the Kc value for the A Protein binding reaction.
Calculate the Kc value for the B -protein binding reaction. Calculate Kc for this reaction. I just need an equation to help me start this problem.
Each molecule in the diagram represents 01. Calculate Kp at 359 K. At 359 K this reaction has a Kc value of 00516.
Calculate the value of Kc for the reaction. What are you looking for. A sample of HI 930 10-3 mol was placed in an empty 200 L container at 1000 K.
Share It On Facebook. 2 NOg Br2g ---- 2NOBr g using the following information. How do you calculate the KC of a.
Looking for an answer to the question. 300 moles A and 200 moles B was placed in a 200 L flask ar reach equilibrium. 2 NOg Br 2 g ---- 2NOBr g using the following information.
Xg 2Yg Zg Calculate Kp at 359 K. The following diagram represents a reaction shown going to completion. G Gibbs Free Energy K Equilibrium Constant and Q Reaction Quotient are related as follows.
School University of California Irvine. Where a mole of reactant A.

Ice Table Practice Problems Initial Concentration Equilibrium Concentration Kc Part 1 Youtube

Properties Of The Equilibrium Constant Video Khan Academy
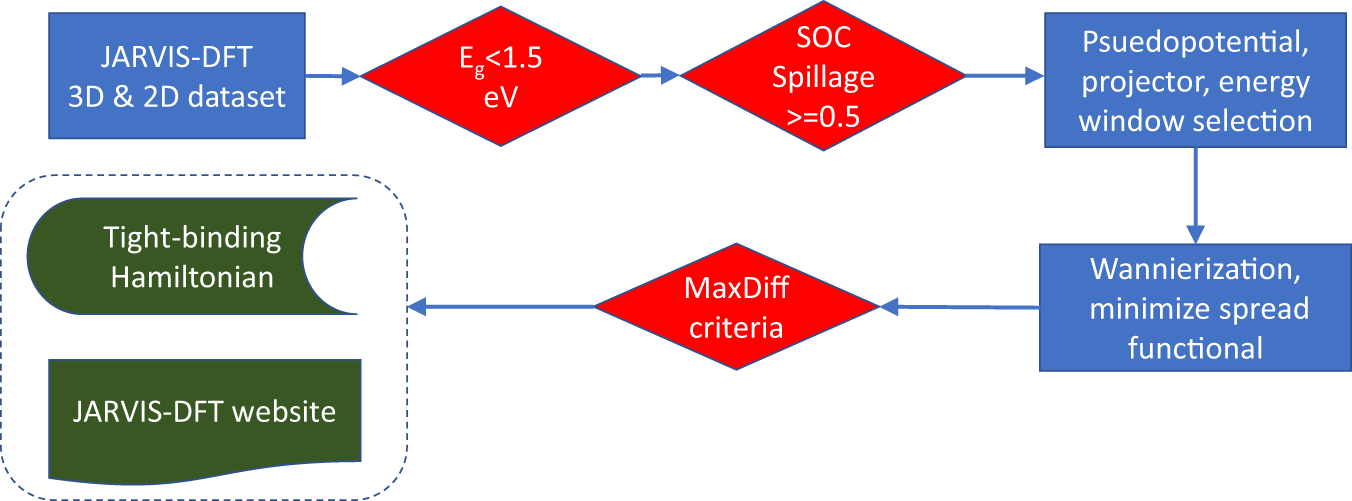
Database Of Wannier Tight Binding Hamiltonians Using High Throughput Density Functional Theory Scientific Data
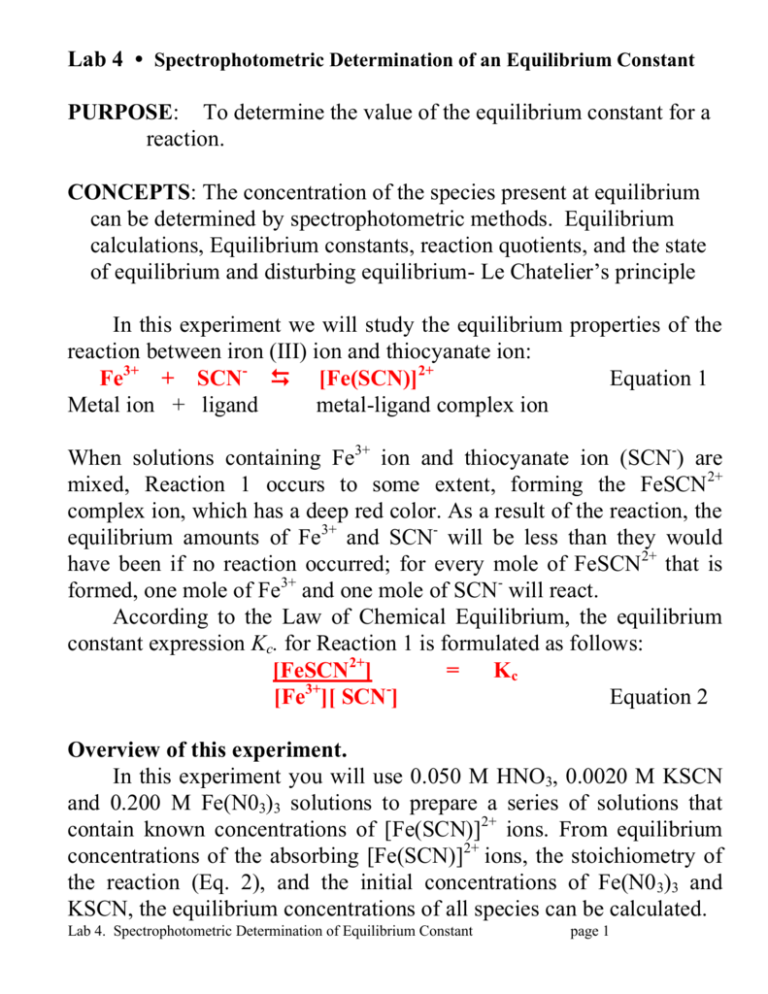
Purpose To Determine The Value Of The Equilibrium Constant For A
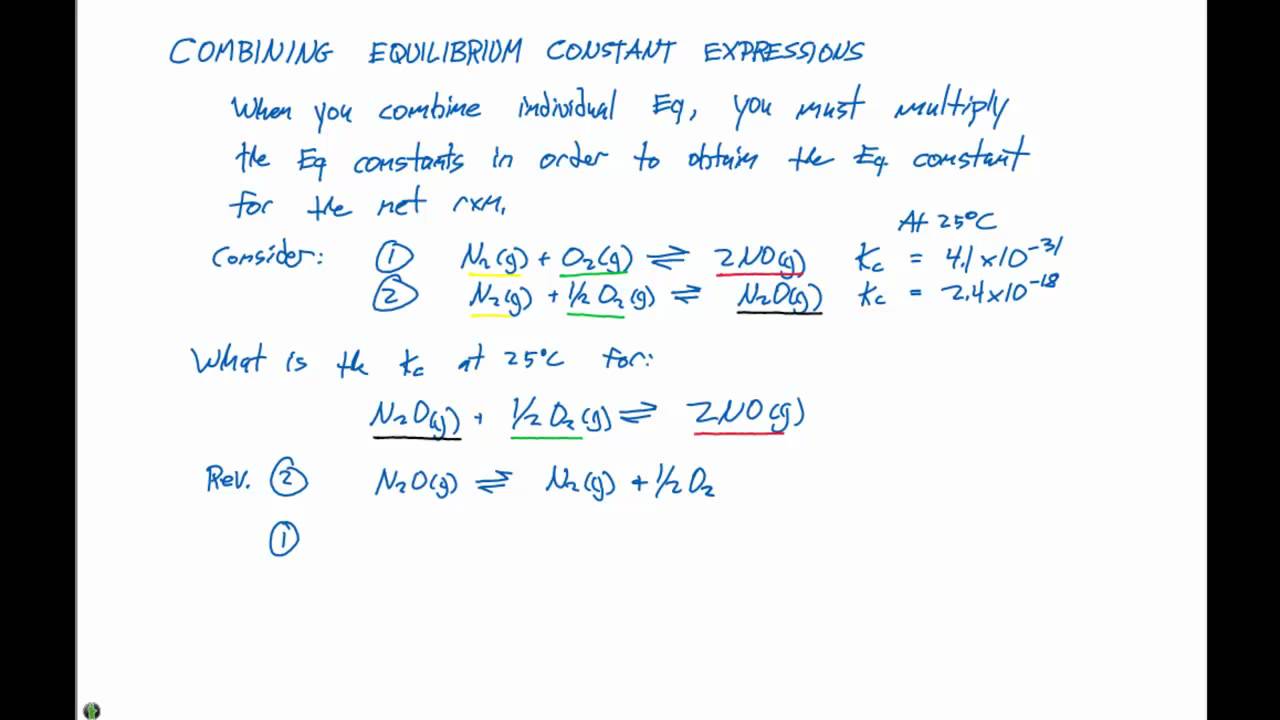
15 3 Combining Equilibrium Constants Youtube
16 4 Acid Strength And The Acid Dissociation Constant Ka Chemistry Libretexts

13 4 Equilibrium Calculations Chemistry
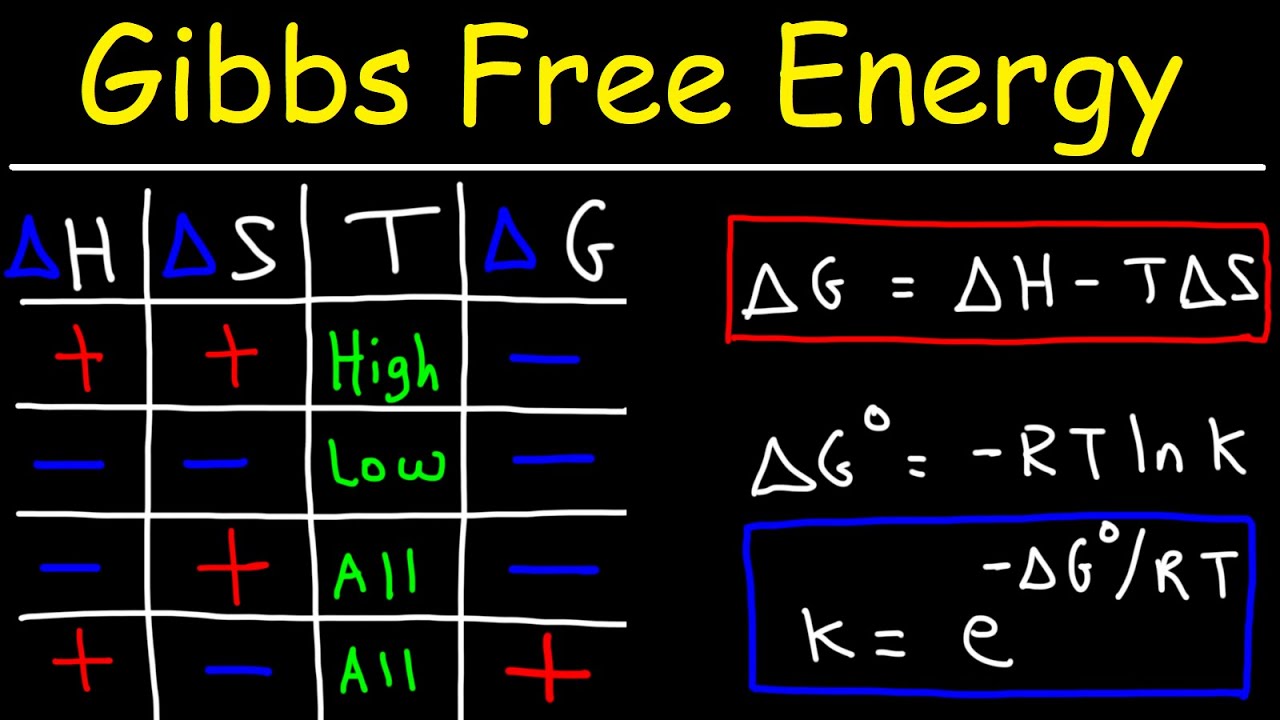
Gibbs Free Energy Entropy Enthalpy Equilibrium Constant K Youtube
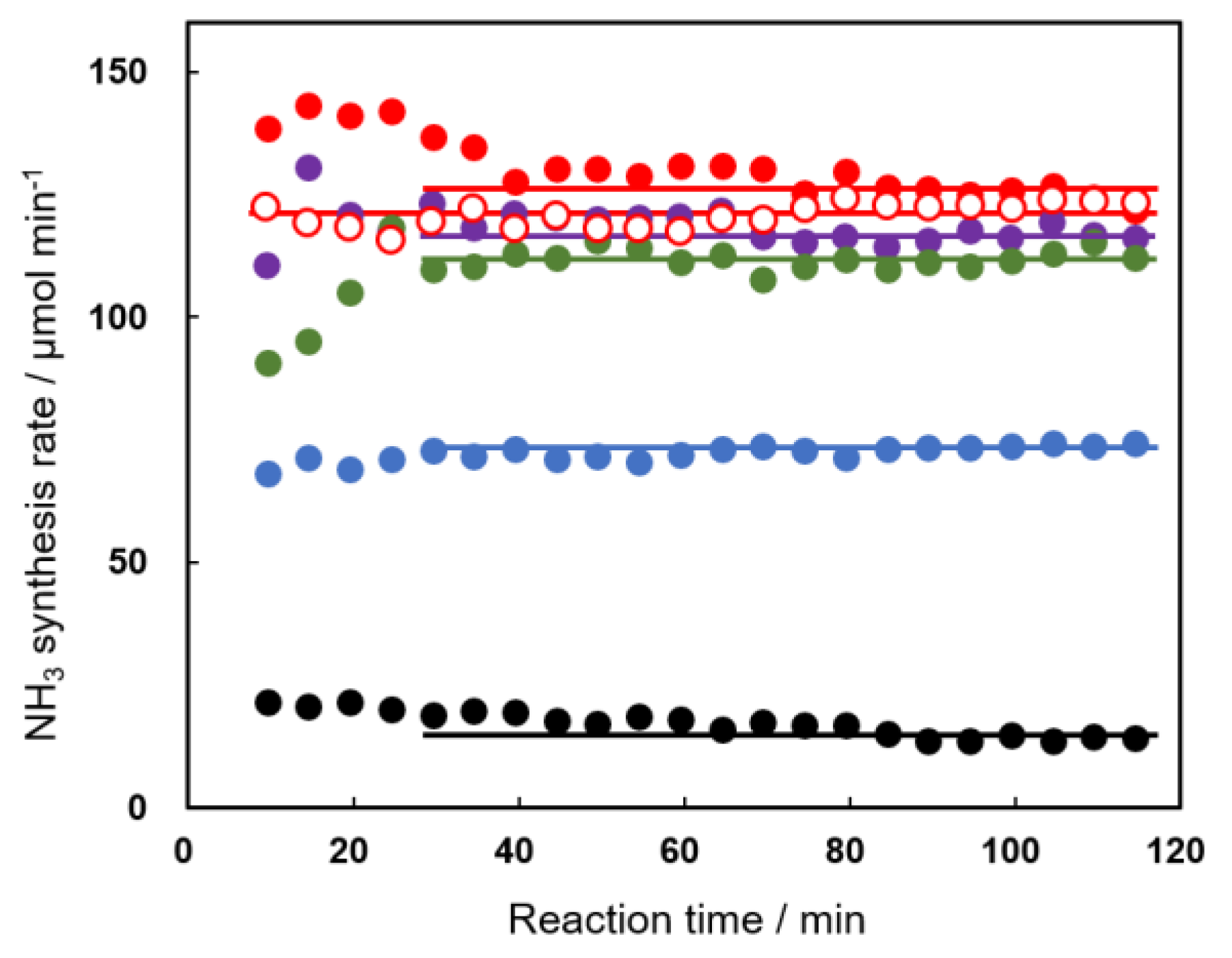
Catalysts Free Full Text Higher Activity Of Ni G Al2o3 Over Fe G Al2o3 And Ru G Al2o3 For Catalytic Ammonia Synthesis In Nonthermal Atmospheric Pressure Plasma Of N2 And H2 Html

At 473 K Equilibrium Constant Kc For Decomposition Of Phosphorus Pentachloride Pcl5 Is 8 3 10 3 If Decomposition Is Depicted As Pcl5 G Pcl3 G Cl2 G Drh 124 0kjmol 1
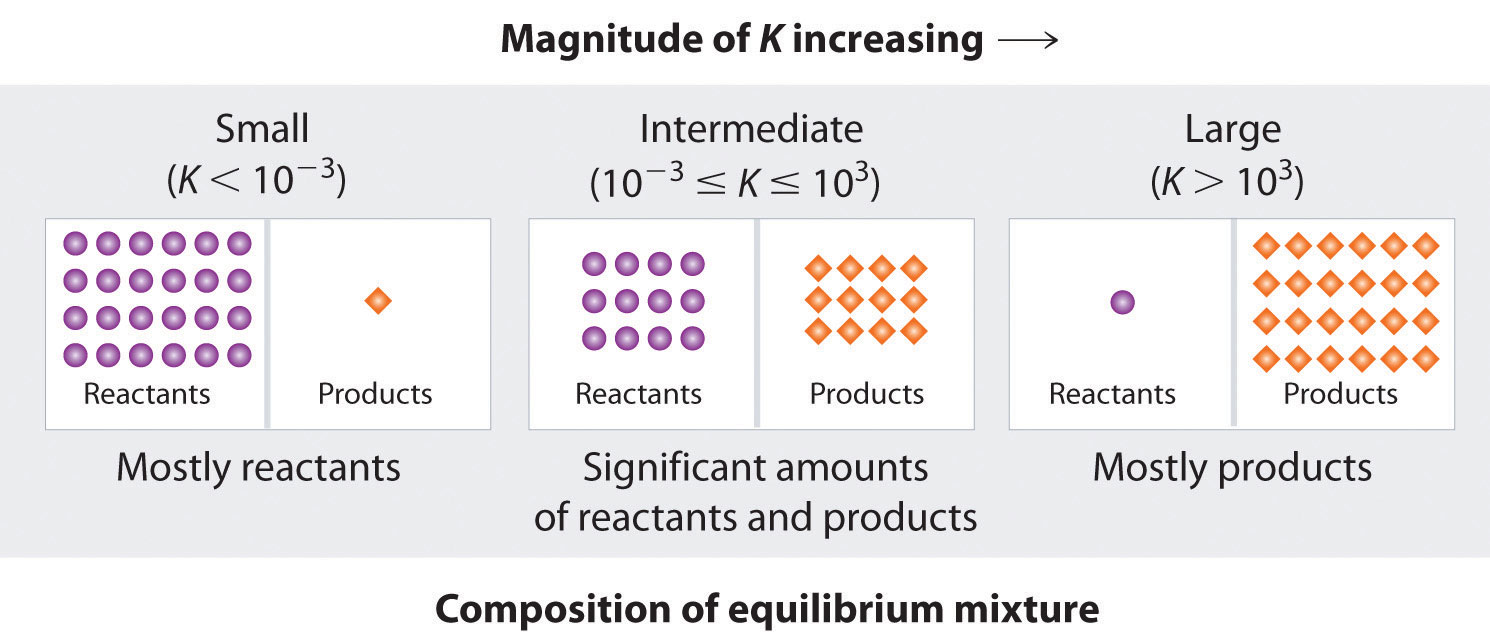
1 2 The Equilibrium Constant Expression Chemistry Libretexts

Interfacial Reactions In Inorganic All Solid State Lithium Batteries Zheng 2021 Batteries Supercaps Wiley Online Library

Catalysts Free Full Text Higher Activity Of Ni G Al2o3 Over Fe G Al2o3 And Ru G Al2o3 For Catalytic Ammonia Synthesis In Nonthermal Atmospheric Pressure Plasma Of N2 And H2 Html

The Reaction 2a G B G 3c G D G Is Begun With The Concentrations Of A And B Both At An Initial Value Of 1 00 M When Equilibrium Is
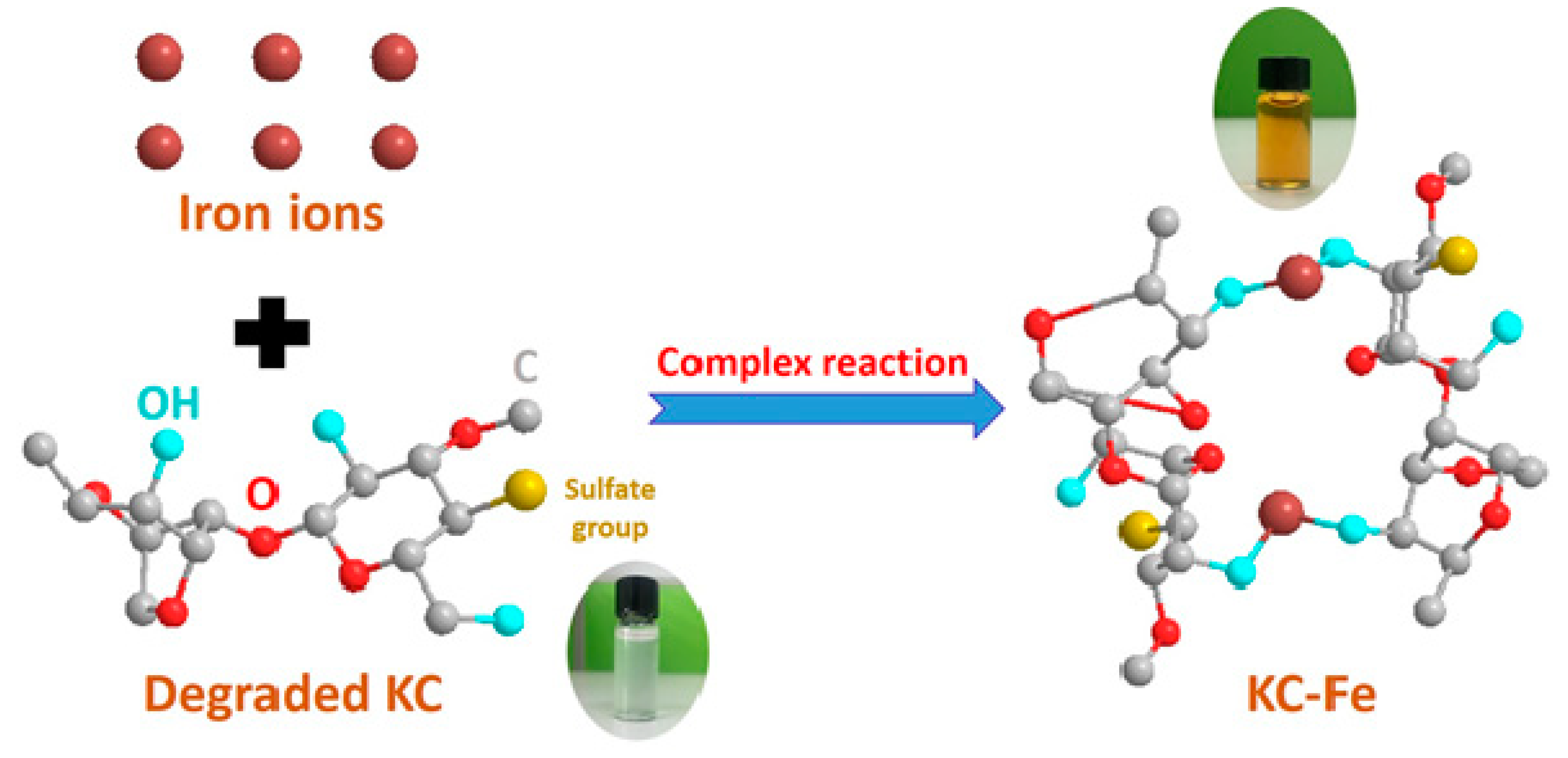
Polymers Free Full Text Synthesis Of A Carrageenan Iron Complex And Its Effect On Flame Retardancy And Smoke Suppression For Waterborne Epoxy Html
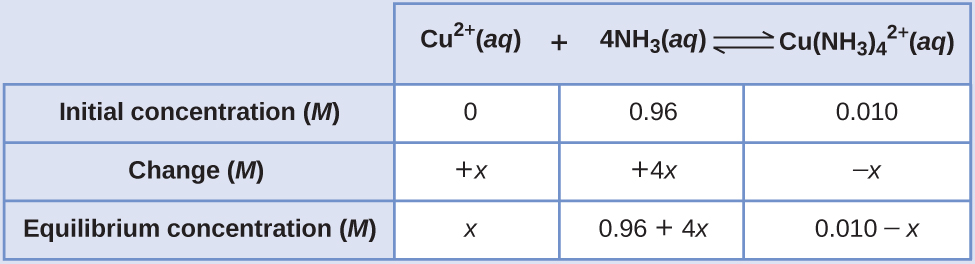
13 4 Equilibrium Calculations Chemistry

At 473 K Equilibrium Constant Kc For Decomposition Of Phosphorus Pentachloride Pcl5 Is 8 3 10 3 If Decomposition Is Depicted As Pcl5 G Pcl3 G Cl2 G Drh 124 0kjmol 1

Writing Equilibrium Constant And Reaction Quotient Expressions Video Khan Academy
Comments
Post a Comment